CRISPR screening is a widely used technique in biological research, offering a powerful approach for identifying genes associated with a biological process and investigating gene function. By introducing whole-genome or pathway-specific gRNA libraries into cell populations, researchers can examine on a large scale how specific genetic perturbations affect cellular behavior under various conditions, including drug treatment, competitive growth, and viral infection.
Pooled CRISPR screening
In a pooled CRISPR screen, individual vectors carrying different gRNAs, and Cas9 if needed, are designed, cloned, and introduced to groups of target cells in vitro or in vivo. A selective pressure is then introduced to the experimental group, and cells are screened for the desired behavior: for instance, positive or negative selection for viability or marker-based selection. High-throughput sequencing and data analysis then identify essential genes, regulatory pathways, and molecular networks involved in these responses. The diagram below demonstrates the workflow of CRISPR-based knockout screens. Different types of CRISPR screening, such as CRISPR knockout (KO), CRISPRa, and CRISPRi, offer distinct approaches and applications tailored to specific research goals. Collectively, these will expand our understanding of cellular processes and create new opportunities for addressing complex biological and disease-related challenges.
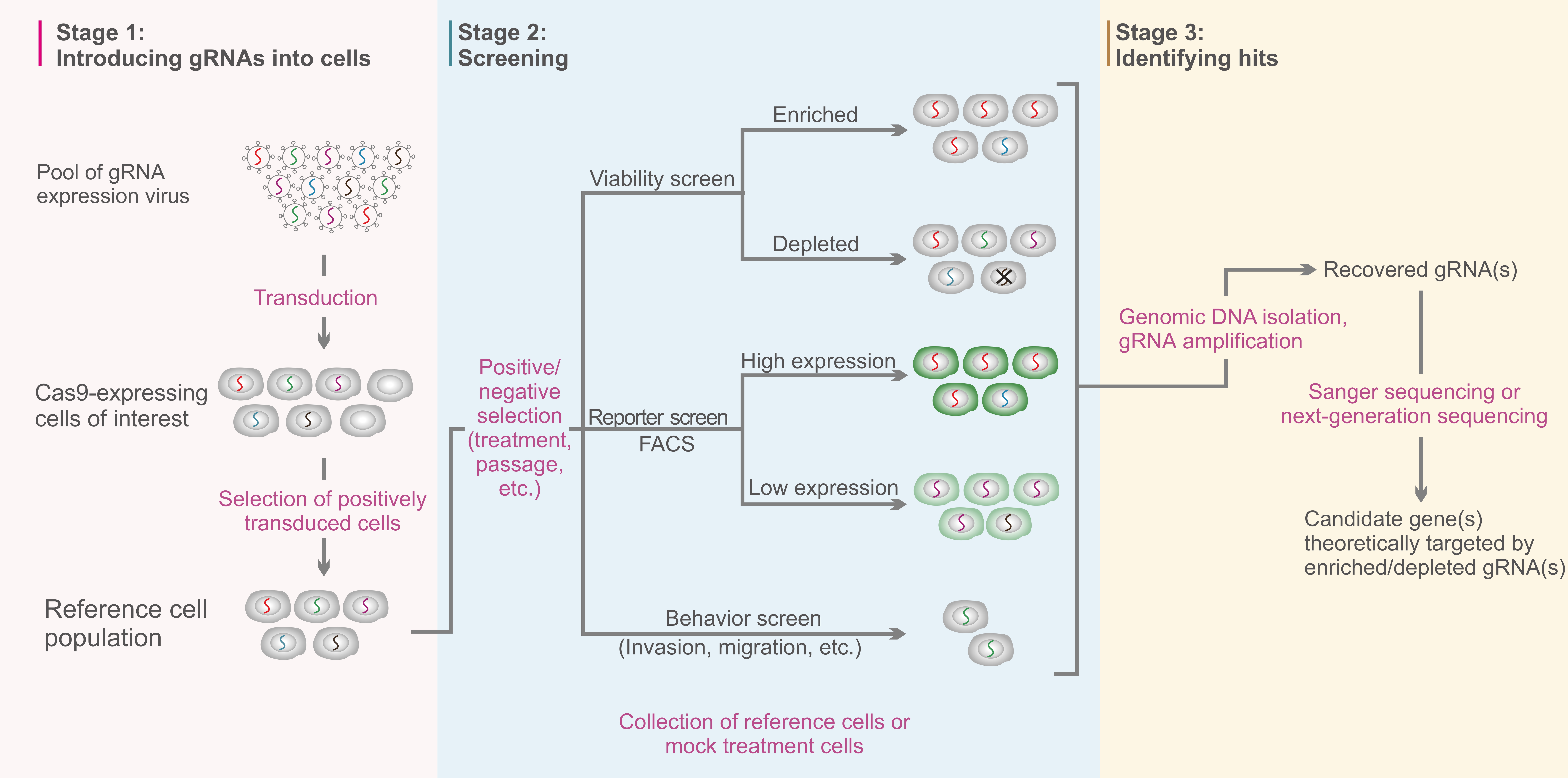
Figure 1. Workflow of CRISPR-based knockout screens. Adapted from Acta Biochim Biophys Sin 44:103-112 (2012).
To ensure that a CRISPR library screen is set up for success, several key considerations must be addressed from the very beginning. Part 1 of this blog post series will focus on the early stages of a library screening experiment, including selecting an appropriate biological system and designing a suitable library specific to your application. The next posts in this series will provide guidance on screening strategies and readout methods.
Biological system
Many decisions about your CRISPR library, including whether to use viral or non-viral delivery, will depend on your target cells. Screens can be performed both in vitro and in vivo. In vivo CRISPR screens can be broadly classified into direct and indirect approaches. Direct in vivo screens involve delivering gRNA libraries directly into the target organ, enabling perturbation of cells within their native environment where cell–cell interactions, signaling networks, and tissue architecture are preserved. This strategy provides greater physiological relevance and therapeutic insight compared to in vitro systems. However, direct delivery of libraries into specific target organs in animals can be technically challenging, and scaling up is particularly difficult for larger libraries due to tissue size. To maintain sufficient coverage, pooling samples from multiple animals may be required. In contrast, indirect in vivo screens involve introducing gRNA libraries into cells in vitro, which are subsequently transplanted into living tissues. This approach circumvents many limitations of direct delivery, making it a powerful alternative; however, its application is largely restricted to experimental systems in which cell transplantation is feasible.
In vitro approaches are a popular choice largely due to their simplicity and scalability. They can be conducted in biologically relevant cell types, such as primary immune cells or neuronal cells, with the choice of cell type tailored to the specific research question. Immortalized cancer cell lines are frequently used because they are robust, easy to maintain, and compatible with large-scale screening, making them valuable representative models. During screens, a gRNA library is introduced to cells, commonly via lentivirus at a low MOI to ensure high transduction efficiency, with most cells receiving a single gRNA construct.
To enable genome editing, both the gRNA and the Cas9 protein (or a Cas variant) need to be co-expressed in the target cells or tissues. For direct in vivo screens, transgenic models expressing Cas9, such as Cas9 knock-in mice, are commonly used. Of note, ubiquitous Cas9 expression can lead to off-target cuts, so conditional and/or inducible Cas9 expression is often employed, e.g., with Cre-dependent LSL-Cas9 mice. These models provide a straightforward approach by eliminating the need to introduce Cas9 within the library, which simplifies experimental design. Alternatively, Cas9 can be supplied through vector-based systems before library screening, such as with transposon systems, which allow stable genomic integration and sustained expression.
For in vitro screens or indirect in vivo screens, there are different ways to express Cas9 in target cells. CRISPR screens can be performed on transgenic Cas9-expressing cells, similar to above; otherwise, Cas9 (or a variant) must be delivered to target cells before or during library screening. Cas9 may be delivered with the lentivirus or transposon system, then screened for Cas9 expression before delivery of the gRNA library, or Cas9 may be incorporated into the library design.
Library design
The design of the gRNA library is critical for the success of the screen, and several key considerations must be made, including application, library size, number of gRNAs per target, and vector design.
While CRISPR knockout libraries are commonly used for their strong phenotypes and relative ease of analysis, perturbations are permanent and may disrupt vital functions. In contrast, CRISPRi and CRISPRa use catalytically inactive Cas9 to repress or activate gene transcription, respectively, and are restricted to targeting regulatory regions and tend to have milder effects. However, they have a lower risk of off-target effects and can be used to modulate essential genes and non-coding RNA.
In terms of size, there are two main types of gRNA libraries: 1) species-specific genome-wide libraries, for instance, targeting the entire human or mouse genome, and 2) targeted libraries that focus on specific sets of genes or specific pathways, including those for knockout of transcription factors, metabolic genes, or libraries targeting non-coding regions, such as lncRNA genes. Whole-genome libraries provide an unbiased approach to interrogate gene function across the entire genome. In contrast, smaller, focused libraries are typically designed based on prior knowledge or hypotheses regarding how selection pressure may influence certain pathways or gene sets. While the whole-genome libraries offer a broader potential, they also require significantly more materials, such as a larger number of cells and viral particles, which can affect the feasibility of the screen. Therefore, it is important to strike a balance between the scope of the screen and the resources available. For instance, if a direct in vivo screen is most appropriate, a smaller, more targeted library is likely the best choice.
The number of gRNAs targeting each gene should also be carefully considered when designing a CRISPR screen. Studies have shown that increasing the number of gRNAs per gene leads to a continuous increase in the number of identified hits, with a more pronounced increase observed for up to four gRNAs per gene. In high-efficiency dual-gRNA CRISPR libraries, each gene is redundantly targeted by 4–6 distinct gRNA pairs delivered in separate vectors, in order to enhance the likelihood of successful hit identification.
Library vector design is a critical component of library construction, as it directly influences the overall performance of CRISPR screens. Importantly, if target cells do not express Cas9, then vectors must be designed to express both Cas9 and single or dual gRNAs. While single gRNAs are typically suitable for knockout of standard coding genes, robust knockout can be achieved with dual gRNAs, which provides the chance of deletion of the fragment between the gRNA target regions. For CRISPRi or CRISPRa screens, vectors should be designed with gRNAs targeting promoters or enhancers.
For CRISPR screens, as with other gene delivery experiments, it is important to design vectors with proper selection markers. Dual markers are a popular choice, for instance, a cassette of EGFP and a puromycin resistance gene (Puro), allowing for selection of positively transduced cells via puromycin and visualization through green fluorescence. Figure 2 shows a vector map for the human whole-genome pooled dual gRNA library, which uses a third-generation lentiviral vector system for efficient, stable, and relatively uniform gRNA expression across a wide range of cell types. Of note, the two gRNAs are driven by distinct U6 promoters (human U6 and macaque U6) and are paired with different gRNA scaffolds that differ in sequence but are functionally equivalent. This design minimizes unwanted recombination between the two gRNA cassettes during lentivirus packaging, which could lead to loss of gRNA and compromised knockout, while enabling PCR amplification and NGS sequencing of either or both gRNAs from transduced cells during screening.
Figure 2. Map of the dual-gRNA library vector.
Click to view fully annotated map and sequence of the dual gRNA library vector
While lentivirus is a popular delivery choice in most cases, including for many differentiated target cells like neurons and immune or muscle cells, other viral delivery systems are most appropriate in specific contexts. AAV is a strong choice when performing in vivo screens or when delivery should be targeted to specific cell types. Libraries can also be designed in virtually any viral or non-viral backbone, depending on the target cells and application. Other considerations that are relevant to designing any CRISPR experiment, including target region, Cas enzyme selection, and gRNA design, are covered in our Optimizing CRISPR blog post.
Library preparation
Following the design of your CRISPR library, gRNA oligos must be synthesized and amplified with high fidelity, and cloned into the selected backbone with high efficiency (e.g., the backbone shown in Figure 2). The constructed vectors in the library should then be validated to verify the quality of the library. We recommend performing Sanger sequencing on a number of randomly selected clones as an initial checkpoint to ensure accurate cloning of vector components, followed by full validation of the library through deep sequencing (Figure 3).

Figure 3. Workflow of pooled gRNA library construction and quality controls.
NGS validation is an essential step to ensure the quality of a CRISPR library, as cloning and bacterial growth can lead to uneven amplification of vectors. To evaluate library quality, NGS reads are aligned to the reference gRNA list to determine the read counts for each guide. The resulting data are then analyzed to assess library representation and uniformity. Metrics such as the percentage of perfectly matched reads and the ratio between the 90th and 10th percentiles of the distribution are commonly used to evaluate whether the library exhibits even distribution. The 90/10 ratio compares the read count at the 90th percentile (representing highly abundant gRNAs) to that at the 10th percentile (representing low-abundance gRNAs); a lower ratio indicates a smaller difference in read counts between highly and lowly represented gRNAs, reflecting a more uniform and balanced library. A gRNA library with high coverage (e.g., close to 100%) and uniform distribution (e.g., low 90/10 ratio) is crucial for conducting unbiased and efficient downstream screening.
Pooled CRISPR screening is a powerful approach for studying gene function and biological mechanisms. Its success depends on careful early planning, including the choice of biological system, library design, and screening strategies. Key factors such as library complexity and vector configuration directly influence performance and data quality. Upcoming articles in this series will discuss screening-related topics, such as selection strategies, readout methods, and approaches for data analyses.
References



